Osmolarity Versus Osmolality. Ethanol level.
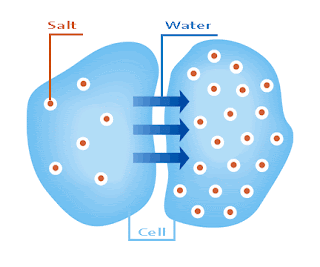
Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides.
The osmolality of plasma is largely a function of Na + concentration and its anions (mainly Cl− and HCO 3−) with contributions from glucose and urea nitrogen. Since each Na + is paired with a univalent anion, the contribution from other cations such as K + , Ca 2+ , and Mg 2+ to the osmolality of plasma is generally not considered. Therefore, the plasma osmolality is calculated by doubling Na + and including the contribution from glucose and urea nitrogen (generally expressed as blood urea
nitrogen or BUN), as follows:
Plasma osmolality (typical in the US) = 2[Na+] + [Glucose]/18 + [ BUN ]/2.8 where [Glucose] and [BUN] are measured in mg/dL.
where 18 and 2.8 are derived from the molecular weights of glucose and urea, respectively. Because serum glucose and urea concentrations are expressed as mg/dL, it is necessary to convert these concentrations to mOsm/L by dividing the molecular weights of glucose (180) or urea nitrogen (28) by 10. Normal serum values are Na + = 142 mEq/L, glucose = 90 mg/dL, and urea nitrogen = 12 mg/dL. The normal range is between 280 and 295 mOsm/kg H2O.
Calculated osmolarity = 2 Na + Glucose + Urea ( all in mmol/L).
The normal range is between 280 and 300 mOsm/l.
If the patient has ingested ethanol, the ethanol level should be included:
calculated osmolality:= 2[Na+] +[Glucose]/18 + [ BUN ]/2.8 + [Ethanol]/3.7
Based on the molecular weight of ethanol the divisor should be 4.6 but empiric data shows that ethanol does not behave as an ideal osmole.