Mechanisms of General Anaesthetic Action
Anatomical Sites of Action
General anaesthetic agents affect both brain and spinal cord to account for physiological responses to nociception, loss of consciousness and inhibition of explicit memory. Auditory and sensory evoked potential data implicate the thalamus as the most likely primary target, but secondary sites such as the limbic system (associated with memory) and certain cortical areas are also
important. Halogenated volatile anaesthetics appear to have a greater influence on the spinal cord than do the intravenous agents.Molecular Theories
At the beginning of the twentieth century, Overton and Meyer independently described the linear correlation between the lipid solubility of anaesthetic agents and their potency (see Figure 1). This correlation was so impressive, given the great variation in structure of these agents, that it suggested a non-specific mechanism of action based on this physicochemical property. Later interpretation pointed out that any highly lipophilic area was a potential site of action, with cell membranes being the most likely contender, given the high concentration of lipids. There are problems with a unified theory based on lipid interactions: some general anaesthetics, such as ketamine, are extreme outliers, and the stereoisomers R-etomidate and S-etomidate have identical lipid solubility but only R-etomidate has anaesthetic properties.
Membrane Lipids
There are several potential lipophilic sites in cell membranes, including the lipid bilayer itself and the annular lipids surrounding ionic channels. Initially it was suggested that anaesthetic agents could penetrate the bilayer and alter the molecular arrangement of the phospholipids, which led to expansion of the membrane and disruption of the function of membrane-spanning ionic channels. Calculations identifying the volume of anaesthetic agent required to expand membranes led to a ‘critical volume hypothesis’. Against such a theory, a 1°C rise in temperature increases membrane thickness to a similar extent as that seen with volatile agents, yet increased temperature does not enhance anaesthesia – the opposite is true. A further theory suggested anaesthetic agents act at specific lipid site(s). The composition of phospholipids in the immediate vicinity of ion channels is different from that of the general lipid bilayer. This proposed disruption of annular lipids associated with specific ion channels led to the perturbation theory. A rapid advance in receptor protein identification within the central nervous system (CNS) has led to newer theories based on interactions with specific proteins. It now seems likely that the correlation between potency and lipid solubility reflects the lipophilic nature of specific protein-based binding site(s).
Protein Site(s) of Action
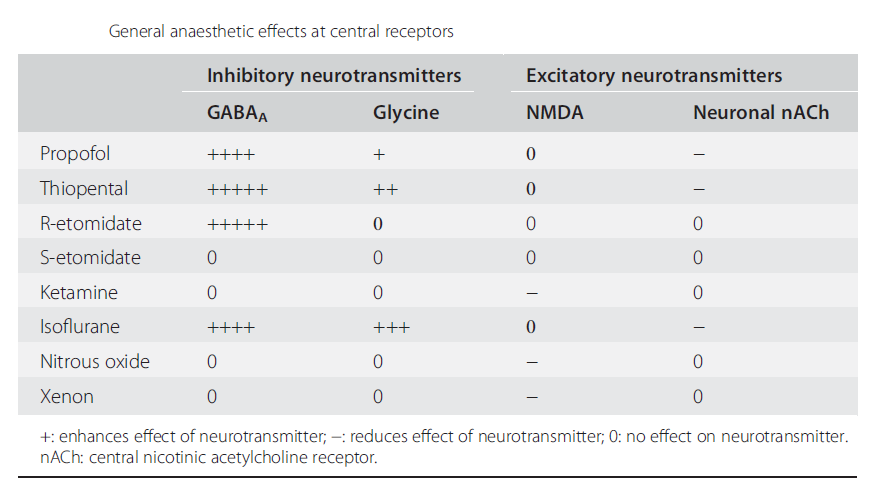
Ligand-gated ionic channels are more sensitive to the action of general anaesthetics than are voltage-gated channels. The interaction at inhibitory (GABAA and glycine) and excitatory (neuronal nicotinic and NMDA) channels have all been studied. Table below summarises the relative activity of a number of agents at these receptors.
GABAA Receptor
The GABAA receptor, like the nicotinic acetylcholine receptor, belongs to the pentameric family of ligand-gated ion-channel receptors. It has binding sites for GABA associated with α subunits and modulatory sites at the α/γ interface for benzodiazepines and on the β subunit for etomidate, barbiturates, propofol and volatile agents (see Figure 2). The stereospecificity of the action of etomidate, presented as an enantiopure preparation of the R(+) isomer, supports the protein-based action of anaesthetics. The S(−) form of etomidate is clinically inactive and at the GABAA receptor there is a 30-fold difference in activity.
Anaesthetics increase channel opening time, so allowing for increased chloride entry resulting in hyperpolarisation. The effect is seen for etomidate, propofol and barbiturates, as well as halogenated volatiles. Etomidate is selective for the GABAA receptor but propofol will also increase glycine channel opening time and is inhibitory at neuronal nicotinic and 5HT3 receptors. The site(s) on the GABAA receptor associated with anaesthetic action are associated with the β subunit and are distinct from the benzodiazepine receptor site. There are at least 30 types of GABAA receptor, each with different subunit composition; β2 and β3 subunits are more sensitive to the effects of etomidate than is the β1 subunit.
Glycine Receptor
The major inhibitory transmitter in the spinal cord and brain stem is glycine, which is associated with a chloride channel similar to the GABAA receptor. The volatile anaesthetics all markedly potentiate the action of glycine, although there is no evidence of stereoselectivity.
It has been suggested that the spinal cord is an important site of action for volatile rather than intravenous anaesthetic agents. Efficacy here correlates more with immobility than awareness.
NMDA Receptor
Neuronal signalling may also be reduced by inhibition of excitatory pathways. The NMDA receptor is involved in long-term signal potentiation associated with learning and memory; it is activated by glutamate, modulated by magnesium and inhibited in a non-competitive manner by ketamine, nitrous oxide and xenon. This glutamate-mediated mechanism represents an additional pathway for anaesthesia. Other anaesthetic agents, such as barbiturates, can reduce the effectiveness of glutamate but at a lower potency than for inhibition of GABAA receptor function.